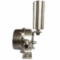Description
Sepha Multi-Q Leak Testing system for rigid and flexible packaging including vials, pouches, sachets, bottles, bags and medical devices. Sepha offers Container Closure Integrity Testing methods with the multi-functional Multi-Q. The standalone, non-destructive leak test system can be used in combination with interchangeable test attachments to leak test vials, ampoules, pre-filled syringes and bags.
In addition to Vacuum and Pressure Decay methods, the Multi-Q is also compatible with a Flexible Membrane, Ramp to Event, Ramp to Proof Pressure and Occlusion attachment, offering an all-in-one solution to test the integrity of all types of pharmaceutical containers including sachets, pouches, bags, trays, medical devices and modified atmospheric packaging (MAP).
The lab-scale unit has a small footprint and offers the flexibility to switch between test methods depending on product and specified test requirements. The Container Closure Integrity Testing methods can be easily changed and the relevant recipes for the selected attachment will be available to start testing immediately. The non-destructive and deterministic test methods provide quick, reliable and repeatable results enabling customers to improve the accuracy of their leak detection procedures and reduce cost as the methods prevents the packaging from being damaged.
Sepha Multi-Q Leak Testing Features
Flexible membrane
- Non-destructive seal and leak detection device designed for flexible or semi-flexible packaging
- Using hybrid membrane technology, the flexible head utilizes the ASTM F2338-09(2013) test method to perform the leak test
- Can detect all defect types including channel leaks, weak seals and pin holes down to 15µm
- Tool-less
- Can test packages that contain tablets/capsules/devices/powders
- Ideal for testing pouches, sachets and bags
- 21 CFR Part 11 compliant
Vacuum decay
- Non-destructive, deterministic quantitative test method
- Utilizes vacuum decay to identify leaks and channel leaks as low as 5µm
- Ideal for testing bottles, ampoules, vials, BFS strips & bottles, medical devices, Pre-filled syringes, pouches and trays
- Can test containers with a water based liquid, protein/glucose, lyo/powder content
- Rapid test time as low as 10 seconds
- Utilizes the ASTM F2338-09(2013) test method to perform the leak test
- 21 CFR Part 11 compliant
Pressure decay
- Non-destructive, deterministic, quantitative test method
- Utilizes pressure decay to identify leaks and channel leaks as low as 5µm
- Ideal for testing vials, ampoules and bottles
- Can test containers with a toxic product, oil based liquid, lyo/powder content
- Rapid test time as low as 10 seconds
- 21 CFR Part 11 compliant
Ramp to event
- Destructive, deterministic, quantitative test method
- Utilizes pressure/vacuum to ramp to a failure event
- Ideal for component testing to detect weak welds/seals etc.
Ramp to proof pressure
- Non-destructive, deterministic, quantitative test method
- Utilizes pressure/vacuum to ramp to a target pressure
Occlusion
- Non-destructive, deterministic, quantitative test method
- Utilizes pressure drop/time
- Typically used to test parts that must remain open to function correctly
Reference : sepha.com
Other Article:
- Brainchild Ramp Soak Controllers
- Schreiner LPC 528
- Sepha Leak Testing
- AW Lake HM High Pressure Turbine Flow Meter
- Hydraulic Test Analyzer AW Lake
- Static Ground Monitoring System with SS Ground Clamp SLA-S-Y
- APG DCR-1006A Ultrasonic Display Controller
- APG RST-5003 Series Web Enabled Display Control Modules
- APG DDX Explosion Proof Digital Panel Meter
- APG DDL Series Large Digital Panel Meter